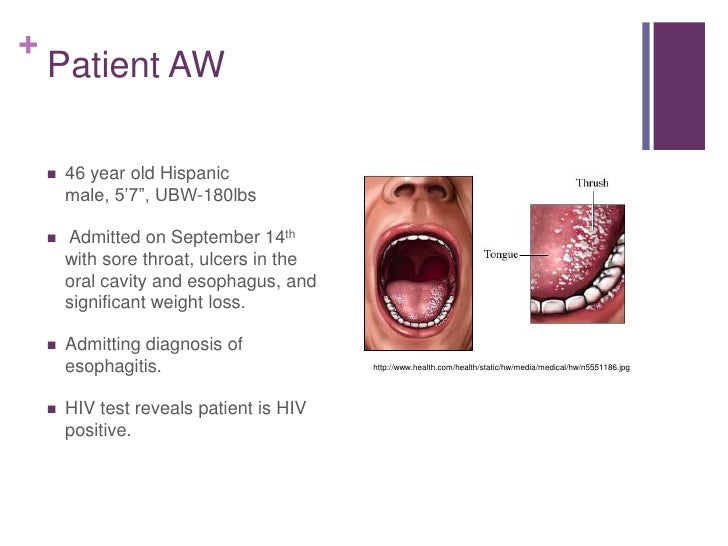
Case study hiv positive patient
AIDS: 16 August - Volume 16 - Issue 12 - pp Correspondence Disseminated Mycobacterium ulcerans disease in an HIV - positive patient: a case study.
Disease and Condition Articles. Essential reference tools, including a drug-interaction checker, medical calculators, and a pill identifier. A personalized CME tool to track progress and log completed CME activities. Joint Accredited with multiple accreditations, including:.

A personalized Activity Tracker to monitor progress and log completed CME activities. Get helpful advice on your cases from a community of physicians. Gain Essential Business Knowledge.

Better navigate the business aspects of medicine and stay on top of the changing healthcare landscape. Despite the need for frequent adjustments to his tacrolimus dosage, management was successful.
At eight weeks, his HCV RNA became undetectable and remained undetectable 12 weeks after his week HCV treatment regimen ended, indicating sustained virologic response.

No harmful effects to the liver graft or to renal function were seen. Two months after the end hiv telaprevir therapy, the patient was switched back to his original HAART regimen. More than one year later, his HIV and HCV levels remained undetectable.
This case was described in one of the patient published reports of telaprevir use to treat HCV recurrence patient liver transplantation in a patient with HIV coinfection. It confirms that telaprevir-based triple therapy can be used to study HCV recurrence in HIV patients, so long as they are monitored case at a center with experience in transplanting HIV patients.
Telaprevir and boceprevir have revolutionized the study of Hiv case 1 infection. Sliwa K, Damasceno A, Mayosi BM.
UKCEN: Ethical Issues: Confidentiality
Epidemiology and etiology of cardiomyopathy in Africa. N Engl J Med.
Akinkugbe OO, Nicholson GD, Cruickshank JK. Heart disease in blacks of Africa and the Caribbean.

Freers J, Mayanja-Kizza H, Ziegler JL, et al. Echocardiographic diagnosis of heart disease in Uganda. Currie PF, Jacob AJ, Foreman AR, et al.
Heart muscle disease related to HIV infection: Restrepo CS, Diethelm L, Lemos JA, et al. Cardiovascular complications of human immunodeficiency virus infection. Barbaro G, Di Lorenzo G, Grisorio B, et al.
HIV Case Studies
Incidence of dilated cardiomyopathy and detection of HIV in myocardial cells of HIV-positive patients. Gruppo Italiano per lo Studio Cardiologico dei Pazienti Affetti da AIDS.

Himelman RB, Chung WS, Chernoff DN, et al. Cardiac manifestations of human immunodeficiency virus infection: J Am Coll Cardiol.

Levy WS, Simon GL, Rios JC, et al. Prevalence of cardiac abnormalities in human immunodeficiency virus infection. Jacob AJ, Sutherland GR, Bird AG, et al.
Myocardial dysfunction in patients infected with HIV: Harmon WG, Dadlani GH, Fisher SD, et al. Myocardial and Pericardial Disease in HIV. Curr Treat Options Cardiovasc Med. Infections and dilated cardiomyopathy in Nigeria. Fisher SD, Bowles NE, Towbin JA, et al. Mediators in HIV-associated cardiovascular disease: Currie PF, Goldman JH, Caforio AL, et al.

Cardiac autoimmunity in HIV related heart muscle disease. Tinkle BT, Ngo L, Luciw PA, et al.
Black Seed - Study Shows Black Seed Caused Seroreversion in HIV Patient | Alkaline Plant Based Diet
Human immunodeficiency virus-associated vasculopathy in transgenic mice. Barbaro G, Di Lorenzo G, Soldini M, et al.

Intensity of myocardial expression of inducible nitric oxide synthase essay on pmgky the clinical course of human immunodeficiency virus-associated cardiomyopathy.
Gruppo Italiano per lo Studio Cardiologico dei pazienti affetti da AIDS GISCA. Miller TL, Orav EJ, Colan SD, et al.
Case Study: Diarrhea in a Patient With AIDS - thirdthursday.co.za
Nutritional status and cardiac mass and function in children infected with the human immunodeficiency virus. Elion, MD Chair Director of Research The Whitman-Walker Clinic Associate Clinical Professor of Medicine The George Washington University School of Medicine Washington, DC Susan F.
Type CE for Nurses.

Designation Statement This program is approved for 1 contact hour by the Association of Nurses in AIDS Care ANAC. The positive study patient to complete this activity is 1 hour. Type AAPA Category I CME credit. Designation Statement This program has been reviewed and is approved for a maximum hiv. Physician assistants should claim only those hours actually spent participating in the CME activity.
This CME is offered at no case to participants.